|
Getting your Trinity Audio player ready... |
Introduction
Advancements in medical diagnostics are transforming how diseases are detected, monitored, and treated. Among the most promising innovations is the liquid biopsy test – a minimally invasive diagnostic method that analyzes biomarkers found in bodily fluids such as blood, urine, or saliva. Liquid biopsy tests are rapidly reshaping clinical practice, particularly in oncology, by offering faster, safer, and more precise insights into disease progression.
Definition
Liquid biopsy tests are minimally invasive diagnostic procedures that analyze biological fluids – most commonly blood – to detect and monitor disease by identifying biomarkers such as circulating tumor DNA (ctDNA), RNA, proteins, or circulating cells. They are widely used in oncology to help detect cancer, assess treatment response, identify genetic mutations, and monitor disease progression or recurrence without the need for surgical tissue biopsies.
What Are Liquid Biopsy Tests?
A liquid biopsy test is a diagnostic procedure that detects disease-related genetic material or biomarkers circulating in body fluids. Unlike traditional tissue biopsies, which require surgical removal of tissue samples, liquid biopsies typically involve a simple blood draw. These tests can identify components such as:
- Circulating tumor DNA (ctDNA)
- Circulating tumor cells (CTCs)
- RNA fragments
- Proteins and exosomes
Because tumors shed DNA and cells into the bloodstream, liquid biopsy tests allow clinicians to detect and analyze cancer-related changes without invasive procedures.
How Liquid Biopsy Tests Work
Liquid biopsy tests work by isolating and analyzing small fragments of DNA or cells released into the bloodstream by tumors or diseased tissues. Advanced molecular techniques such as next-generation sequencing (NGS) and polymerase chain reaction (PCR) are then used to identify genetic mutations, biomarkers, or molecular signatures associated with specific diseases.
This approach provides real-time insights into the genetic landscape of a disease, making it especially useful for tracking changes over time.
Key Advantages of Liquid Biopsy Tests
1. Minimally Invasive and Safer
Traditional tissue biopsies can be painful, risky, and sometimes impossible due to tumor location. Liquid biopsy tests require only a blood sample, reducing discomfort, complications, and recovery time for patients.
2. Early Detection of Disease
Liquid biopsy tests can detect minute traces of disease markers, often before symptoms appear or tumors are visible through imaging. This early detection is critical for improving treatment outcomes, particularly in cancer.
3. Real-Time Disease Monitoring
Because liquid biopsies can be performed repeatedly, they enable continuous monitoring of disease progression, treatment response, and relapse. This is especially valuable for assessing how tumors evolve over time.
4. Personalized Treatment Decisions
By identifying specific genetic mutations, liquid biopsy tests help clinicians tailor treatments to individual patients. This supports the growing field of precision medicine, where therapies are customized based on a patient’s unique genetic profile.
5. Overcoming Tumor Heterogeneity
Tumors are often genetically diverse, and a single tissue biopsy may not capture all mutations. Liquid biopsy tests sample DNA shed from multiple tumor sites, providing a more comprehensive view of the disease.
Applications of Liquid Biopsy Tests
Cancer Diagnosis and Management:
Liquid biopsy tests are most widely used in oncology. They help detect cancers such as lung, breast, colorectal, prostate, and ovarian cancer. These tests are also instrumental in:
- Identifying actionable mutations
- Monitoring treatment effectiveness
- Detecting drug resistance
- Identifying cancer recurrence earlier than imaging
Prenatal Testing:
Liquid biopsy technology is used in non-invasive prenatal testing (NIPT) to detect genetic abnormalities in fetuses by analyzing fetal DNA circulating in the mother’s blood.
Organ Transplant Monitoring:
Liquid biopsy tests can detect donor-derived DNA in transplant recipients, helping clinicians identify early signs of organ rejection without invasive biopsies.
Infectious and Chronic Diseases:
Researchers are exploring the use of liquid biopsy tests to monitor infectious diseases, autoimmune conditions, and chronic illnesses by detecting disease-specific biomarkers.
Limitations and Challenges
Despite their many benefits, liquid biopsy tests are not without challenges. Some limitations include:
- Sensitivity issues: Early-stage diseases may release very small amounts of biomarkers, making detection difficult.
- False negatives or positives: Results may require confirmation through traditional diagnostic methods.
- Cost and accessibility: Advanced sequencing technologies can be expensive and may not be widely available in all healthcare settings.
- Regulatory and standardization hurdles: Clinical adoption requires rigorous validation and regulatory approval.
Ongoing research and technological advancements continue to address these challenges, improving accuracy and affordability.
Future Trends of Liquid Biopsy Tests Market
Rising Demand for Early Cancer Detection:
The liquid biopsy tests market is expected to grow significantly due to increasing demand for early and non-invasive cancer detection. Healthcare providers are prioritizing screening methods that improve survival rates by identifying cancer at earlier stages.
Advancements in Genomic Technologies:
Continuous improvements in next-generation sequencing (NGS), digital PCR, and bioinformatics are enhancing the sensitivity and accuracy of liquid biopsy tests. These technological advancements are expanding their clinical applications and reliability.
Growth of Precision and Personalized Medicine:
Liquid biopsy tests are playing a key role in precision medicine by enabling personalized treatment selection based on genetic profiling. This trend is driving adoption across oncology and targeted therapy programs.
Expansion Beyond Oncology:
While oncology remains the dominant application, future market growth will be supported by expanding use in prenatal testing, organ transplant monitoring, and chronic disease management.
Increasing Investment and Regulatory Approvals:
Rising investments from biotechnology companies and increasing regulatory approvals for liquid biopsy-based diagnostics are accelerating market expansion and global adoption.
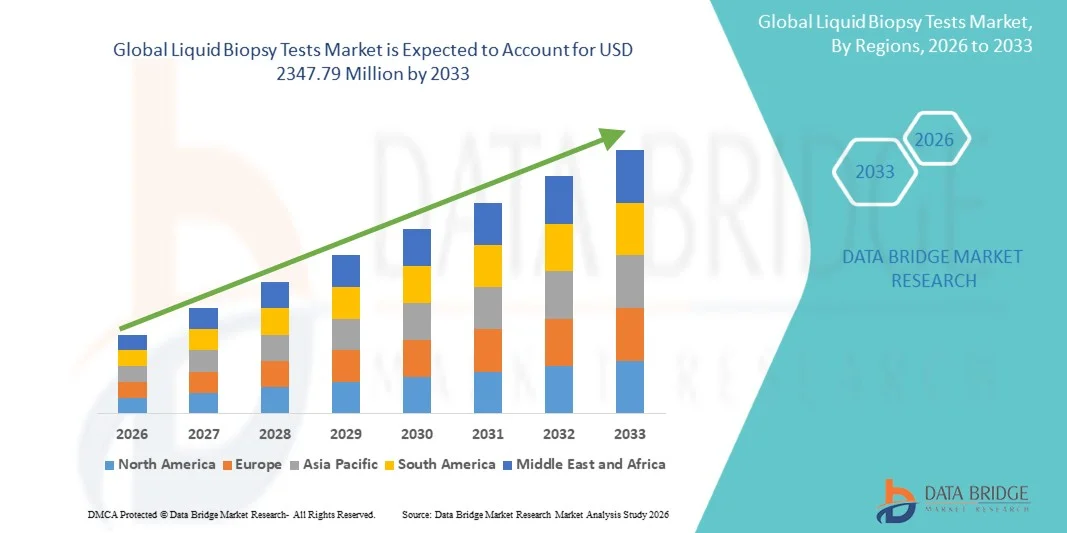
Growth Rate of Liquid Biopsy Tests Market
According to Data Bridge Market Research, the liquid biopsy tests market was estimated to be worth USD 673.2 million in 2025 and is projected to grow at a compound annual growth rate (CAGR) of 16.90% to reach USD 2347.79 million by 2033.
Learn More: https://www.databridgemarketresearch.com/reports/global-liquid-biopsy-tests-market
Conclusion
Liquid biopsy tests represent a groundbreaking shift in diagnostic medicine. By offering a minimally invasive, accurate, and dynamic approach to disease detection and monitoring, they empower clinicians to make better-informed decisions and improve patient outcomes. While challenges remain, continued innovation and research are rapidly expanding the clinical applications of liquid biopsy technology.